The Role of Air Pollution in the Decline and Excess Mortality
of Oaks and Hickories in the Eastern U.S.
William B. Grant, Ph.D.
Ch. 12. The Effects of Acidic Deposition on Pennslyvania's Forests, 1999, W.E. Sharpe and J.R. Drohan, editors, Proceedings of the 1998 Pennslyvania Acidic Deposition Conference, Volume 1, Environmental Resources Research Institute, University Park, PA, pp 151-160
ABSTRACT
The ecologic study approach is used to find links between acid deposition and ozone dose for oak decline and excess mortality of oaks and hickories. In this approach, USDA Forest Service Forest Inventory and Analysis (FIA) data at the state level are used with acid deposition and ozone dose data for the latter half of the 1980s. Ozone dose is found to be very highly correlated with oak decline in the southeastern U.S. Excess oak and hickory mortality rates appeared starting in about the mid-1970s and continue to increase today. The excess mortality is thought to be due to the accumulation of years of effects of acid deposition and ozone exposure. In addition, using mortality data in the northeastern U.S., red oaks are found to be most sensitive to ozone, while white oaks are found to be most sensitive to acid deposition.
INTRODUCTION
The ecological approach is used here to link air pollution to decline of oak and excess mortality of oaks and hickories. While the ecological approach generally has not been applied to the study of forests, it can be applied to European forests. If one compares maps of damaged trees in Europe with the modeled nitrate and aluminum concentrations in the forest soils (de Vries et al., 1994), one finds that the highest percentage of trees damaged in 1995 (European Comm., 1996) are found in The Czech Republic, Poland, and Slovakia, which also have the heaviest modeled soil nitrate and aluminum loadings.
PRINCIPAL FINDINGS
Decline of Oaks Correlated with Ozone Dose
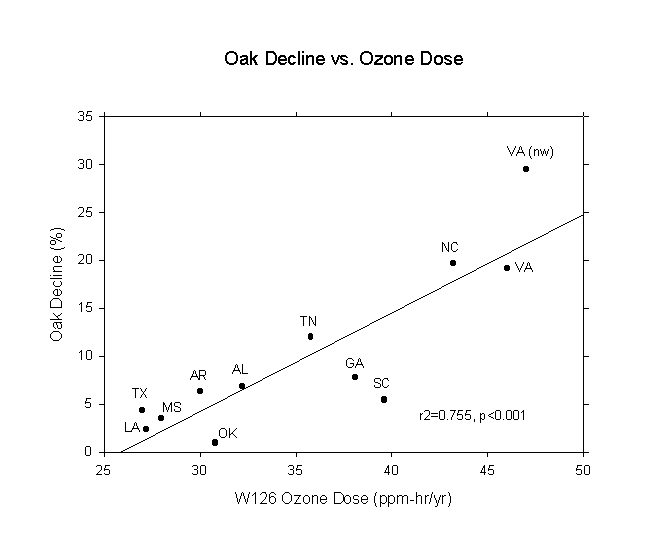
Having read of all the adverse effects of acid ion deposition and ozone exposure, the author was surprised not to find any comprehensive reports in the literature regarding their role in eastern U.S. forests. When he came upon a USDA Forest Service report (Bechtold et al., 1992) showing a strong gradient of upland oak decline from Texas to Virginia for the mid-1980s, he immediately thought that the gradient was caused by air pollution. Affected areas ranged from 0.92% in Oklahoma to 19.63% in North Carolina. A bit later, he found the acid ion deposition maps for 1985-1987 (Sisterson et al., 1990) and ozone W126 dose maps as presented in Allen and Gholz (1996). The acid and ozone maps were used to determine average doses for the forest regions shown in Bechtold et al. (1992). The statistical results are given in Table 1 and the curve is shown in Figure 1. The very high correlation of oak decline with ozone immediately suggested that ozone was, indeed, playing a major role in the decline of oak forests in the southeast U.S., where red oaks dominate over white oaks. It should also be pointed out that a number of recent papers have reported that ozone is likely having an adverse impact on eastern U.S. forests (Hogsett et al., 1997; Lefohn et al., 1997; Ollinger et al., 1997; Chappelka and Samuelson, 1998; Loucks, 1998a,b).
Table 1. Regression statistics for oak decline in the southeastern U.S. in relation to state-averaged acid ion deposition and ozone dose.
_________________________________________________
Pollutant r r2 F p
_________________________________________________
ozone 0.87 0.76 30.8 <0.001
NO3 0.31 0.10 0.7 0.416
SO4 0.65 0.42 5.1 0.058
__________________________________________________
AL, AR, GA, LA, MS, NC, SC, OK, TN, TX, VA (FL omitted)

Figure 1. Oak decline vs. ozone dose for the southeast U.S. in the late 1980s.
Gypsy moths (Lymantria dispar) have been suggested as a factor in the decline of oaks in Virginia. However, only 5,200 acres (2,100 hectares) were affected in Virginia in 1985 (Oak et al., 1991), which was near the time (1986) of the determination of oak decline in Virginia. Since the large decline in oaks in Virginia was measured in 1986, a time when gypsy moth defoliation was minimal, gypsy moths cannot be considered a major cause of decline at that time. Although other factors, such as stand dynamics, due perhaps to oaks replacing chestnuts (Castanea dentata Borkh.) and American elms (Ulmus americana L.), which have largely died out from their range in the eastern U.S. due to introduced fungal diseases, could explain the results, it seems unlikely. Most of the dieback occurred in the early part of the twentieth century, so oaks that replaced them would be 50-80 years old now. Since northern red oaks (Quercus rubra L.) are shown to still be growing rapidly on the way to becoming canopy-dominant trees (Oliver and Larson, 1996), it seems unlikely that they would be experiencing significant decline at this point, especially in a geographical distribution where decline is highly correlated with ozone dose.
Spatial Mortality Patterns Related to Acid and Ozone
The next step was to look at oak mortality rates to see whether geographical distributions correlated with acid ion deposition or ozone dose. The data set used was the USDA Forest Service FIA data for the mid-1980s for the northeast U.S. Although it would have been desirable to use the entire eastern U.S., in the other states, the oak data in the published FIA reports are not separated into red and white oak categories . The data are based on sampling in each state. Every 5-22 years, the Forest Service surveys a number of plots and uses aerial photographs to extrapolate the plot data to the surrounding forests. For this analysis, annual growing stock mortality at the end of the period was divided by growing stock volume at the beginning of the period. The resulting values were then compared statistically with acid ion depositions and ozone exposures suitably averaged over the forest regions for each state.
The statistical results are given in Table 2. The interesting result is that red oaks are strongly affected by ozone but not by acid deposition, while the white oaks are strongly affected by acid deposition but not by ozone. These results are generally consistent with studies of the effects of acid deposition and ozone for each family, including the results for Q. robur L., which is a European oak in the white oak family. For example, Opydo (1996) found that the decline of Q. robur on the Krotoszyn Plateau (west-central part of Poland) is associated with low soil pH (3.8-4.9 in the upper 20 cm of soil not primarily of limestone origin) and high mobile aluminum.
Additional support for this finding, while merely anecdotal, comes from Berlin in 1996. The author visited the Berlin Botanical Gardens and came upon a pair of 100-year old North American oaks. The red oak was in excellent health, but the white oak showed signs of decline, with leaves missing from several branches. Since acid ion deposition is a more serious problem in Berlin than is ozone exposure (see, e.g., Schulze et al. (1989) and Landmann and Bonneau (1995) for discussions of the amounts and relative importances of acid deposition and ozone in Germany and France, respectively) the two oaks behaved exactly as expected from geographical studies in the U.S.
Table 2. Regression statistics for red oak and white oak (chestnut oak was included with white oak) and hickory mortality rates, based on nearest data prior to 1987 as the center year, for acid ion deposition and ozone dose.
__________________________________________________________
r r2 F p
__________________________________________________________
Red oaks (N=12)*
ozone 0.712 0.507 10.3 0.009
NO3 -0.11 0.012 0.1 0.740
SO4 -0.12 0.013 0.1 0.722
White oaks (N=12)
ozone -0.39 0.151 1.8 0.212
NO3 0.671 0.450 8.2 0.017
SO4 0.642 0.412 7.0 0.025
Hickories (N=17)
ozone 0.611 0.373 8.9 0.009
__________________________________________________________
Oaks: CT, DE, KY, MA, MD, NH, NJ, NY, OH, PA, WV, VT (RI omitted)
Hickories: AL, CT, GA, IA, KY, MD, MI, MO, NC, NY, OH, PA, SC, TN, VA, WI, WV
* N is the number of states included in the analysis.
Temporal Mortality Trends Related to Acidic Deposition and Ozone
Next, following a suggestion of Orie Loucks, trends of oak and hickory mortality were examined for the eastern U.S., again using the USDA-FS FIA data. The statistical results are given in Table 3 and the data are shown in Figures 2 and 3. Note that there is an approximate doubling of the mortality rate between the beginning and end of the study period. The mortality rates correspond roughly to a 200-year life expectancy at the beginning, falling to about a 100-year life expectancy at the end of the period. Note that while the statistical correlations for states with gypsy moths are stronger than those without gypsy moths, the regression line equations for states with and without gypsy moths give very similar results. However, the effects of gypsy moths may only affect the latter years. Similar, but more pronounced, results were also found for hickory (Carya sp.). This is consistent with the idea that gypsy moths acting alone have not been a very strong influence on oak and hickory mortality, but that some more fundamental force must be underlying the trends observed. These results are consistent with increases in both acid ion deposition and ozone exposure due to increases in fossil fuel combustion this century, especially since 1950. The buildup of nitrogen excess in North American ecosystems is considered an adverse factor for forests (Fenn et al., 1998).
Table 3. Temporal trends of oak and hickory mortality in the eastern U.S. using linear regressions.
______________________________________________________________________________
Species Condition r r2 F p equation
______________________________________________________________________________
Oak No gypsy moths* 0.496 0.246 7.8 0.010 -27.042 + 0.0141*year
Gypsy moths** 0.782 0.611 12.6 0.008 -26.417 + 0.0137*year
Hickory No gypsy moths 0.604 0.365 12.1 0.002 -35.308 + 0.0183*year
Gypsy moths 0.972 0.945 68.1 0.001 -45.348 + 0.0233*year
______________________________________________________________________________
* GA, KY, NC, OH, SC, TN, WV
** MD, PA, VA
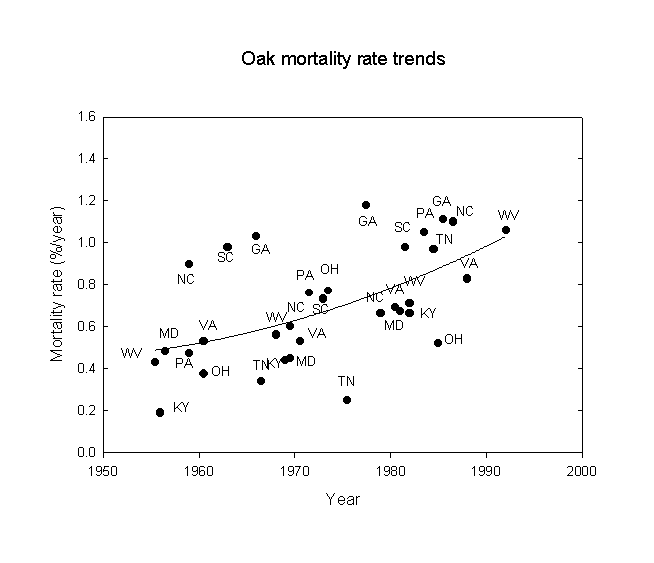
Figure 2. Oak mortality rate trends for southeastern U.S. states, 1950-1996.
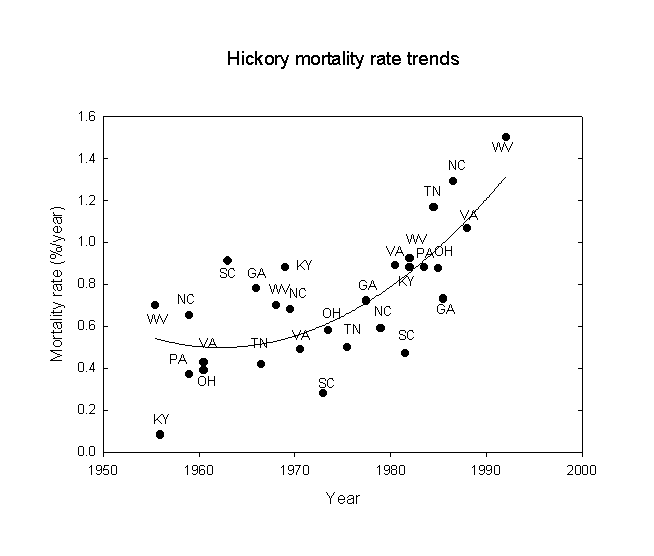
Figure 3. Hickory mortality rate trends for southeastern U.S. states, 1950-1996.
Additional supporting evidence for this conclusion is found in the data on white oak tree ring growth rates. Phipps and Whiton (1988) studied white oak tree rings from a variety of locations in the eastern U.S. They found that growth rates slowed down almost everywhere they looked around 1950. The authors provided no explanation for the finding. This observation is consistent with acid ion deposition increases due to increases in fossil fuel combustion in mid-century, especially since white oaks appear to be sensitive to acid ions.
Oaks and Ozone
Since the finding that oaks, especially Q. rubra, seem to be sensitive to ozone, a brief review of the literature on the effect of ozone on trees in general and Q. rubra in particular seems to be in order. First, a number of recent papers have shown that ozone levels in the eastern U.S. forests are high enough to adversely affect trees (Gilliam and Turrill, 1995; Hogsett et al., 1997; Lefohn et al., 1997; Baumgardner and Edgerton, 1998; Chappelka and Samuelson, 1998). Second, a number of recent papers have shown that mature Q. rubra are significantly more sensitive to ozone doses than are seedlings, which have typically been used to assess the effects of ozone (Samuelson and Edwards, 1993; Samuelson and Kelly, 1996; Samuelson et al., 1996). This result holds even though growth and maintenance respiration in leaves of Q. rubra are similar for seedlings and mature trees (Wullschleger et al., 1996). The difference between the effects of ozone doses on seedlings and mature trees could arise from greater access to soil water, the lack of structurally complex branches in the canopy, large sinks associated with acorn production and tissue maintenance, and nutritional differences (Chappelka and Samuelson, 1998). The net photosynthetic rate (PSR) of Q. mongolica has been shown to decrease rapidly as ozone concentration increases (Kim and Kim, 1997). The PSR was shown to reduce to 75% of normal for ozone concentrations of 60 ppb.
Another problem in trying to assess the impact of ozone on the growth and health of oaks is that ozone exposure may not lead to visible signs of injury on the leaves. More sensitive leaf chemistry analyses can, however, be used to assess impacts [e.g., Kangasjärvi et al., 1994; Schmieden and Wild, 1995]. The impacts may be manifested in the form of reduced defense against biological and abiological stressors, such as drought, frost, fungal diseases, and insects (e.g., Skärby et al., 1998). In summary, there is sufficient information in the peer-reviewed forestry literature to suggest that Q. rubra can be and is being adversely impacted by current ozone doses in the eastern United States that this possibility, raised by the statistical results presented in this paper, should be taken seriously.
Possibility That Other Factors are Involved
Hendershot and Jones (1989) reviewed the maple decline in Quebec, which showed a sudden increase in 1982. They noted that studies of insect defoliation did not find correlations with decline. However, natural disturbances including insects, thaw and droughts were seen as accelerating the decline. Declines were found in the amounts of base cations between 1968 and 1985. The fact that the decline occurs over a wide geographical distribution with a large range of soil types and ecologic settings suggests that only some ubiquitous environmental impact, such as air pollution, is likely to be the cause of the decline.
Oak et al. (1996) developed several oak decline rating models for use in the southeastern U.S. Parameters included soil depth class, oak basal area, site index, stand age, clay content, slope gradient, and site index/age. The r2's determined for various regions varied from 0.65 for the northern Appalachian subregion to 0.30 for the southern Appalachian subregion. The authors conclude that "The relatively low r2 values and variation in the relationships for some attributes suggest that major oak decline events may be influenced by additional factors." Ozone was dismissed as a major factor in oak decline in a superficial manner, essentially saying that no gradient studies had shown an effect for red oak.
What is most consistent with the findings reported here is that air pollution, both directly and by weakening trees, is the largest single agent that can be linked to the decline and excess mortality evident in the USDA Forest Service FIA data trends and geographic distributions. This hypothesis is entirely consistent with the view of the Forest Service expressed by Wargo (1996).
Suggestions for Future Work to Confirm or Refute Our Hypothesis
Horntvedt et al. (1992) outlines the Norwegian monitoring program for forest damage. The program includes monitoring the forest through the use of plots, specific data on individual trees, and soil conditions. In addition, air and precipitation quality are measured on regional scales. Also, the forest condition reports for Europe (European Commission, 1996), in which crown condition for trees is monitored on a 16 km x 16 km transnational grid annually, serves as a good model
CONCLUSIONS
We have used USDA Forest Service Forest Inventory and Analysis data, along with ozone and acid ion deposition doses, to show that red oaks experience decline and increased mortality due to ozone, with some contribution from acid ion deposition, while white oaks show increased mortality due to acid ion deposition. In the author’s model, acid ions change the soil chemistry, leading both to increased N fertilization, but also reduced base cations and increased aluminum. This can lead to reduced root growth, making trees more susceptible to drought conditions. It can also reduce trees' frost hardiness. In addition, it can increase the susceptibility of trees to attacks by fungal infections and insects, both of which utilize the more abundant nitrogen compounds. Ozone attacks leaves, reduces photoproduction, and changes the sugar/starch ratios, resulting in fewer carbohydrates being stored in the roots. As a result, trees are more susceptible to defoliation by insects. In addition, by making the stomata more rigid, ozone can reduce the trees' ability to regulate transpiration, again making them more susceptible to drought.
Although the author cannot prove that other factors may not be giving rise to the spatial and temporal patterns observed in oak decline and mortality, the situation now seems to be analogous to that of the announcement of the discovery of the Antarctic "ozone hole" (Farman et al., 1985). At that time, Farman et al. could show that a temporal change in stratospheric ozone over Halley Bay was matched by the trends in tropospheric chlorofluorocarbons (CFCs). Two expeditions to Antarctica in the following two years were able to determine the mechanisms linking CFCs to the ozone decreases. The author challenges the forest research community either to propose a hypothesis that better explains the results presented here, as well as others found during field and modeling studies, or to accept the hypothesis that both acid ion deposition and ozone are having major adverse impacts on the eastern oak and hickory forests.
ACKNOWLEDGMENTS
The author thanks the USDA Forest Service for providing the reports with the data, especially Thomas Frieswyk, Forest Health Monitoring Program, Radnor, PA; Orie L. Loucks, Zoology Department, Miami University, Oxford, OH, and the late John Flynn for many helpful discussions; and Harvard Ayers, Appalachian Voices, Boone, NC, for encouragement.
LITERATURE CITED
Allen, E.R. and H.L. Gholz. 1996. Air quality and atmospheric deposition in southern U.S. forests, In S. Fox and R.A. Mickler (eds.), Impact of Air Pollutants on Southern Pine Forests, Springer-Verlag, NY. pp. 83-170.
Baumgardner, R.E. and E.S. Edgerton. 1998. Rural ozone across the eastern United States: Analysis of CASTNet data, 1988-1995. J. Air Water Mngt. Assoc. 48: 674-688.
Bechtold, W.A., W.H. Hoffard, and R.L. Anderson. 1992.)Forest Health Monitoring in the South, 1991, USDA Forest Service, Southeastern Forest Experiment Station, Gen. Tech. Rep. SE-81, 40 pp.
Chappelka, A.H. and L.J. Samuelson. 1998. Ambient ozone effects on forest trees of the eastern United States: A review. New Phytol. 139: 91-108.
de Vries, W., G.J. Reinds, M. Posch, and J. Kämära. 1994. Simulation of soil response to acidic deposition scenarios in Europe. Water, Air and Soil Pollut. 78: 215-246.
European Commission, United Nations Economic Commission for Europe. 1996. Forest Condition in Europe, Results of the 1995 Survey, 128 pp plus appendices, Hamburg, Germany.
Farman, J.C., B.G. Gardner, and J.D. Shanklin, 1985. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction, Nature 315: 207.
Fenn, M.E., M.A. Poth, J.D. Aber et al. 1998. Nitrogen excess in North American ecosystems: Predisposing factors, ecosystem responses, and management strategies. Ecological Applications 8(3): 706-733.
Gilliam, F.S. and N.L. Turrill. 1995. Temporal patterns of ozone pollution in West Virginia: Implications for high-elevation hardwood forests. J. Air Waste Mngt. Assoc. 45: 621-626.
Hendershot, W. and A.R.C. Jones. Aug. 1989. Maple decline in Quebec: A discussion of possible causes and the use of fertilizers to limit damage. The Forestry Chronicle 280-287.
Hogsett, W.E., A. Herstrom, J.A. Laurence, J.E. Weber, E.H. Lee, and D. Tingey. 1997. An approach for characterizing tropospheric ozone risk to forests. Environ. Mngt. 21: 105-120.
Hogsett, W.E., J.E. Weber, D. Tingey, A. Herstrom, E.H. Lee, and J.A. Laurence. 1997. Environmental auditing, an approach for characterizing tropospheric ozone risk to forests. Environ. Manage. 21: 105-120.
Kangasjärvi, J., J. Talvinen, M. Utriainen, and R. Karjalainen. 1994. Plant defence systems induced by ozone. Plant, Cell Environ. 17: 783-794.
Kim, J.W. and J.-H. Kim. 1997. Modelling the net photosynthetic rate of Quercus mongolica stands affected by ambient ozone. Ecol. Model. 97: 167-177.
Horntvedt, R., D. Aamlid, A. Rørå, E. Joranger. 1992. Monitoring programme for forest damage. Norwegian J. Agric. Sci., 6: 1-17.
Landmann, G., and M. Monneau (Eds.). 1995. Forest Decline and Atmospheric Deposition Effects in the French Mountains, Springer-Verlag, Berlin.
Lefohn, A.S., W. Jackson, D.S. Shadwick, and H.P. Knudsen. 1997. Effect of surface ozone exposures on vegetation grown in the Southern Appalachian Mountains: Identification of possible areas of concern. Atmos. Environ. 31: 1695-1708.
Loucks, O.L. 1998a. In changing forests, a search for answers. In H. Ayers, J. Hager, and C.E. Little (eds.), An Appalachian Tragedy. Sierra Club Books, San Francisco, CA. pp. 85-97.
Loucks, O.L. 1998b. The epidemiology of forest decline in eastern deciduous forests. Northeastern Naturalist 5(2): 1443-154.
Oak, S.W., C.M. Huber, and R.M. Sheffield. 1991. Incidence and Impact of Oak Decline in Western Virginia, 1986. USDA Forest Service, Resource Bulletin SE-123. 16 pp.
Oak, S.W., F. Tainter, J. Williams, and D.A. Starkey. 1996. Oak decline risk rating for the southeastern United States. Ann. Sci. For. 53: 721-730.
Oliver, C.D. and B.C. Larson. 1996. Forest Stand Dynamics, Updated Edition. John Wiley & Sons, New York.
Ollinger, S.V., J.D. Aber, and P.B. Reich. 1997. Simulating ozone effects on forest productivity: Interactions among leaf-, canopy-, and stand-level processes. Ecol. Applic. 7: 1237-1254.
Opydo, J. 1996. Estimation of aluminium pollution in oak stands on the Krotoszyn Plateau. Acta Soc. Botanicorum Poloniae 65(3-4): 345-348.
Phipps, R.L. and J.C. Whiton. 1988. Decline in long-term growth trends of white oak. Can. J. For. Res. 18: 24-32.
Samuelson, L.J. and G.S. Edwards. 1993. A comparison of sensitivity to ozone in seedlings and trees of Quercus rubra L. New Phytol. 125: 373-379.
Samuelson, J.J. and J.M. Kelly. 1996. Carbon partitioning and allocation in northern red oak seedlings and mature trees in response to ozone. Tree Physiology 16: 853-858.
Samuelson, J.J., J.M. Kelly, P.A. Mays, and G.S. Edwards. 1996. Growth and nutrition of Quercus rubra L. seedlings and mature trees after three seasons of ozone exposure. Environ. Pollut. 91: 317-323.
Schmieden, U. and A. Wild. 1995. The contribution of ozone to forest decline. Physiologia Plantarum 94: 371-378.
Schulze, E.-D., O.L. Lange, and R. Oren. 1989. Forest Decline and Air Pollution. Springer-Verlag, Berlin.
Sisterson, D.L., V.C. Bowersox, T.P. Meyers, A.R. Olsen, R.J. Vong, J.C. Simpson, and V. Mohnen. 1990. NAPAP Report 6, Deposition Monitoring: Methods and Results, Government Printing Office, Washington, DC.
Skärby, L, H. Ro-Poulsen, F.A.M. Wellburn, and L.J. Sheppard. 1998. Impacts of ozone on forests: a European perspective. New Phytol. 139: 109-122.
Wargo, P.M. 1996. Consequences of environmental stress on oak: Predisposition to pathogens. Ann. Sci. For. 53: 359-368.
Wullschleger, S.D., P.J. Hanson, and G.S. Edwards. 1996. Growth and maintenance respiration in leaves of northern red oak seedlings and mature trees after 3 years of ozone exposure. Plant, Cell Environ. 19: 577-584.
. . . to Eco Systems' Home Page
Contact Gerry Hawkes: ghawkes@eco-systems.com